ACUTE INFLAMMATION
Immuno-Cardiology
The process of cardiac transplantation results in the liberation of large amounts of extracellular RNA into the plasma. This RNA drives an inflammatory cascade of cytokines and thrombosis which damages the transplanted organ leading to graft dysfunction or in some cases rejection. Convincing data from pre-clinical animal models of heart transplantation has demonstrated that treating the transplanted organ with RNase decreases the associated cardiac damage and increases graft survival.
Ma, G., et al. Ribonuclease alleviates hepatic ischemia-reperfusion injury by suppressing excessive cytokine release and TLR3-mediated apoptosis in mice, Cytokine, 133, (155178), (2020).
Stieger, P., et al. Targeting of Extracellular RNA Reduces Edema Formation and Infarct Size and Improves Survival After Myocardial Infarction in Mice, Journal of the American Heart Association, 6, 6, (2017).
Ischemic and Hemorrhagic Stroke
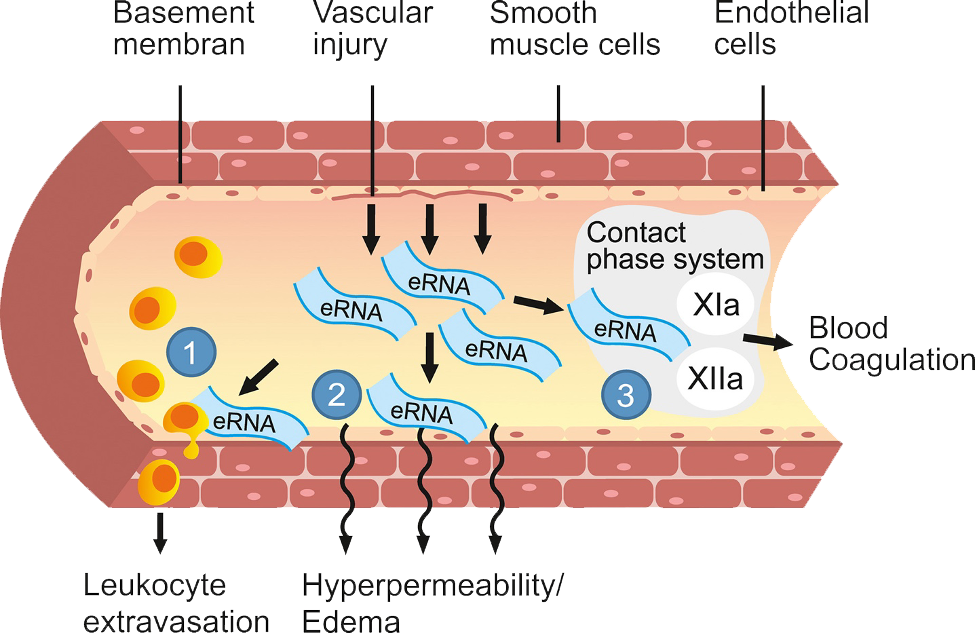
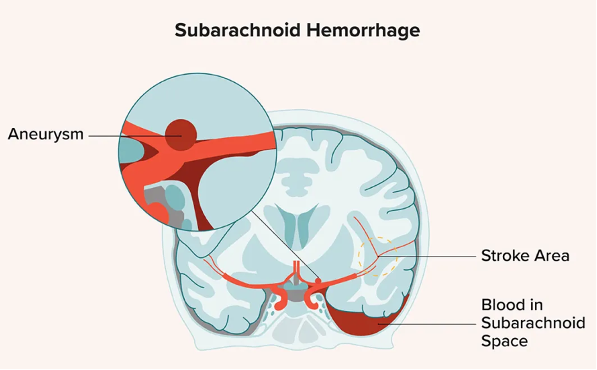
Ischemic stroke and subarachnoid hemorrhage are acute, life-threatening emergencies with high mortality. Immediate medical or surgical interventions to reverse hypoxia or stop bleeding are lifesaving. However, in many patients there is a secondary increase in brain damage due to post-acute inflammation that is mediated in part by extracellular RNA. In preclinical studies in mice, the extent of this damage was reduced by RNase treatment, suggesting a potential role for RSLV-132 in limiting the extent of neurological damage in stroke.
CHRONIC INFLAMMATION
NETosis Related Inflammation
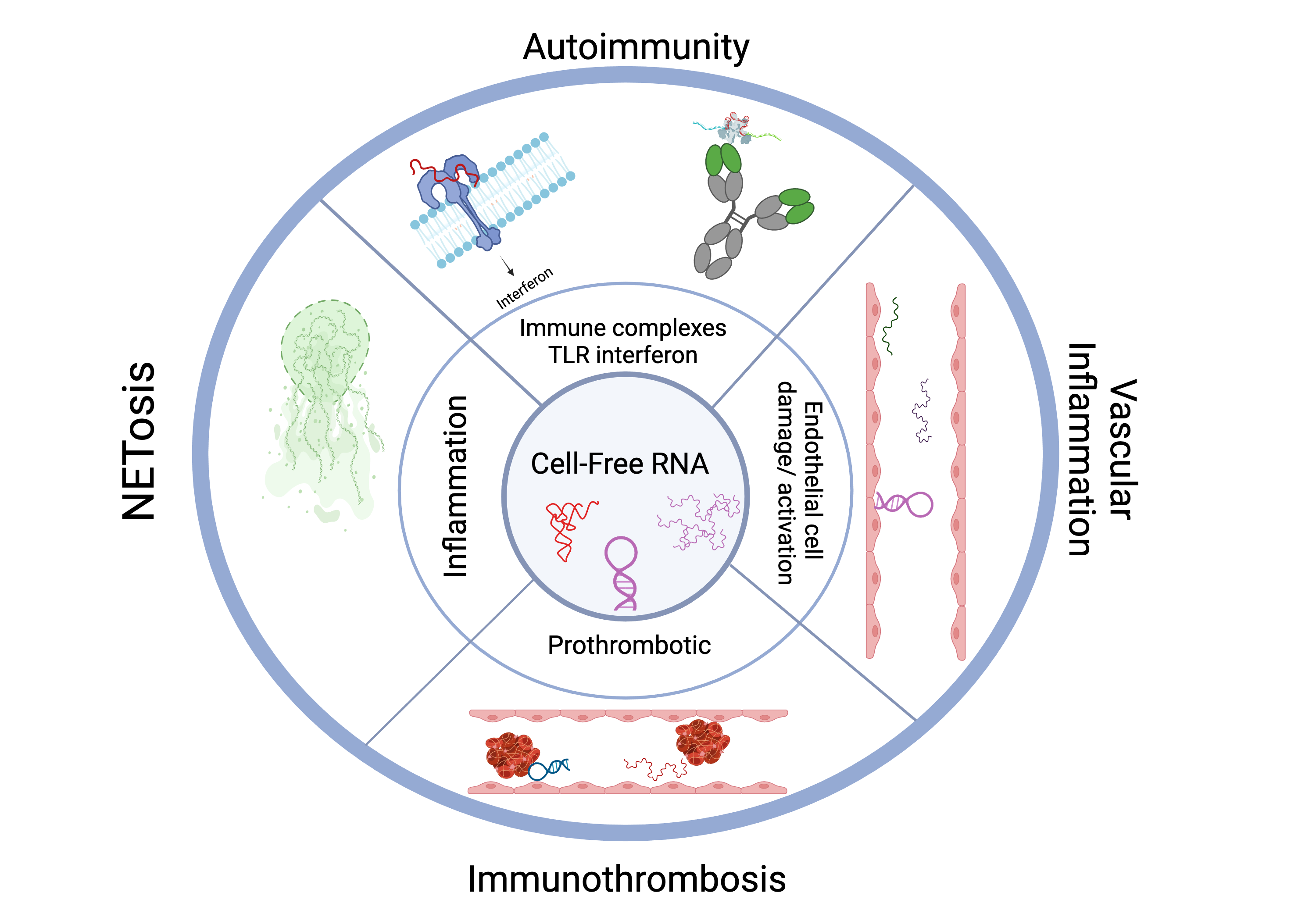
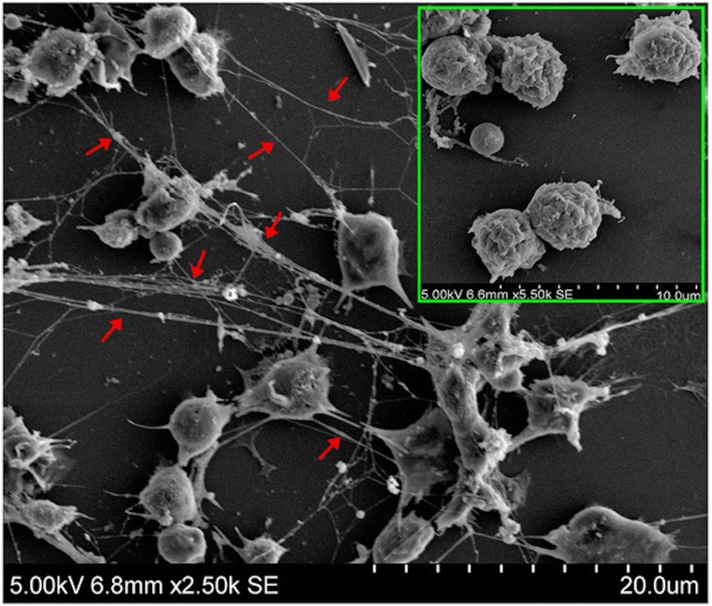
Neutrophil Extracellular Traps (NETs) are molecular scaffolds comprised of a mixture of DNA and RNA that are extruded from neutrophils at sites of inflammation. NETs contain autoantigens and recruit inflammatory mediators to the site of inflammation. They are recognized as key drivers in acute and chronic inflammatory diseases. Targeting NET-related RNA, DNA or both represent attractive therapeutic targets.
For example, hidradenitis suppurativa (HS) is a severe dermatologic disease with unmet medical need. Multiple studies have demonstrated a causative role of NETs in HS. Similar findings have been published in psoriasis.
RSLV-145 is a bi-specific Fc nuclease fusion protein with both RNase and DNase enzyme activity. Targeting two key components of NETs simultaneously with RSLV-145 is a promising approach to eliminate NET-driven inflammation.
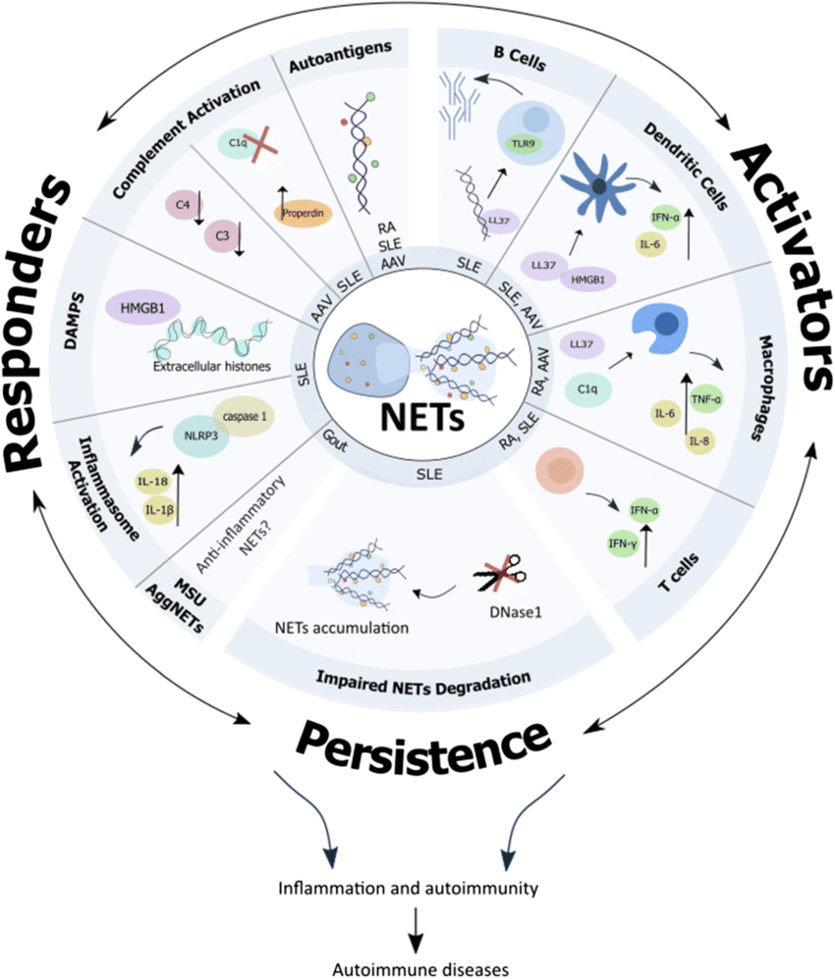
Hanata, N. and Kaplan, M.J. The role of neutrophil extracellular traps in inflammatory rheumatic diseases. Curr Opin Rheumatol, 2024, 36. Doi:10.1097/BOR.00000001054
Herster, F., et al. Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nature Communications (2020) 11:105.
Lood C, et al. Neutrophil traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22(2):146-53.
Zhang, J. et al. NETosis of psoriasis: a critical step in amplifying the inflammatory response. Front. Immmunol. 31 July 2024
Autoimmune Diseases
Systemic Lupus Erythematosus (SLE) and Sjogren’s Syndrome are characterized by the presence of chronic inflammation driven by cytokines such as Type I Interferons. Circulating RNA-containing autoantibodies and cell-free RNA molecules are significant drivers of this interferon response. RNase1 treatment improved lupus in an animal model and in pilot clinical trials, RSLV-132 led to improvement in symptoms and clinical activity in these autoimmune diseases.
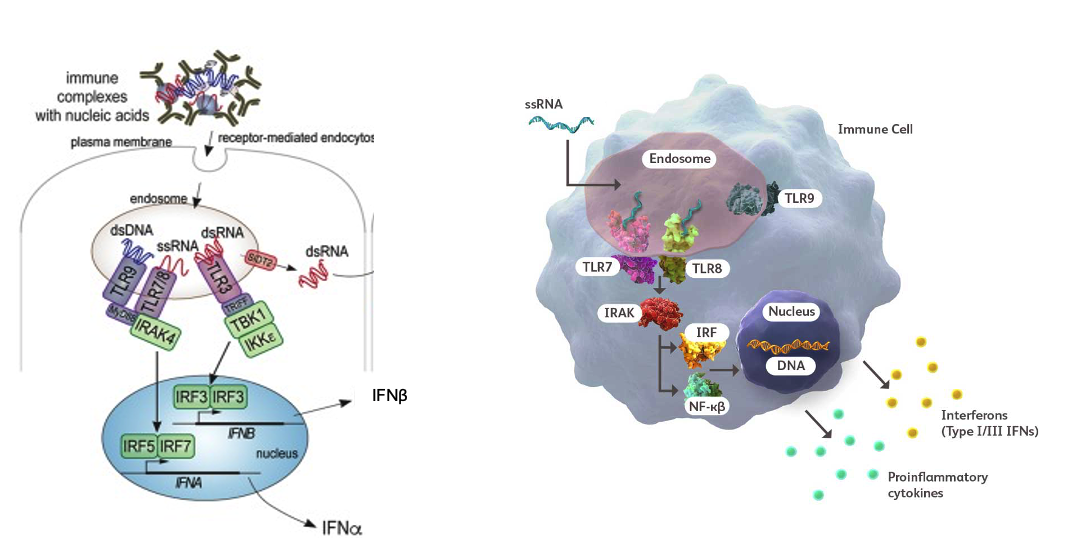
Burge, D.J. et al. Evaluation of RNase therapy in systemic lupus erythematosus: a randomized phase 2a clinical trial of RSLV-132. Lupus Science and Medicine 2024;11:e001113.
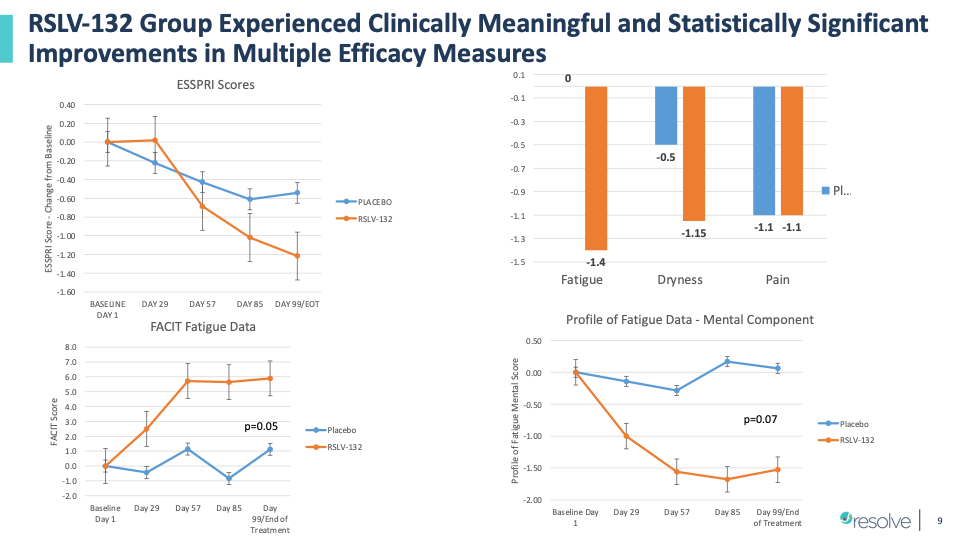
Efficacy Data from Resolve Phase 2 Randomized Clinical Trial in Primary Sjogren’s Syndrome with RNase (RSLV-132)
Post-viral Diseases
Cell free RNA causes endothelial cell dysregulation and a prothrombotic state which may contribute to the endothelial cell dysfunction and immune-thrombosis implicated in the pathogenesis of long COVID and other post viral syndromes, such as myalgic encephalitis/chronic fatigue syndrome (ME/CFS). Data from a phase 2 study of RSLV-132 in long COVID suggest that RNase may simultaneously target multiple key events in the pathogenesis of long COVID and lead to clinical benefit.
