PHASE 1
PHASE 2
PHASE 3
RSLV-132
RSLV-145
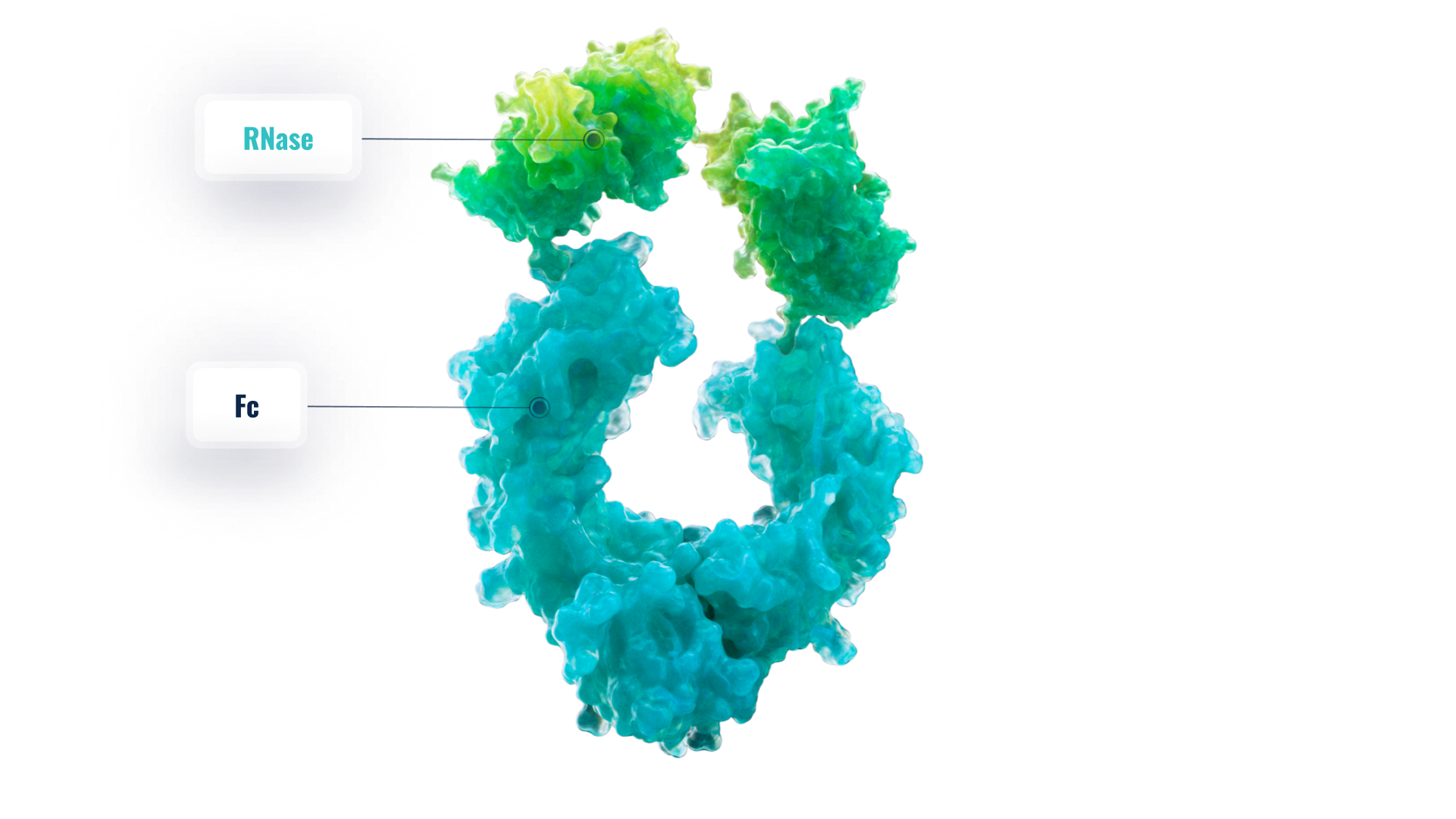
RSLV-132
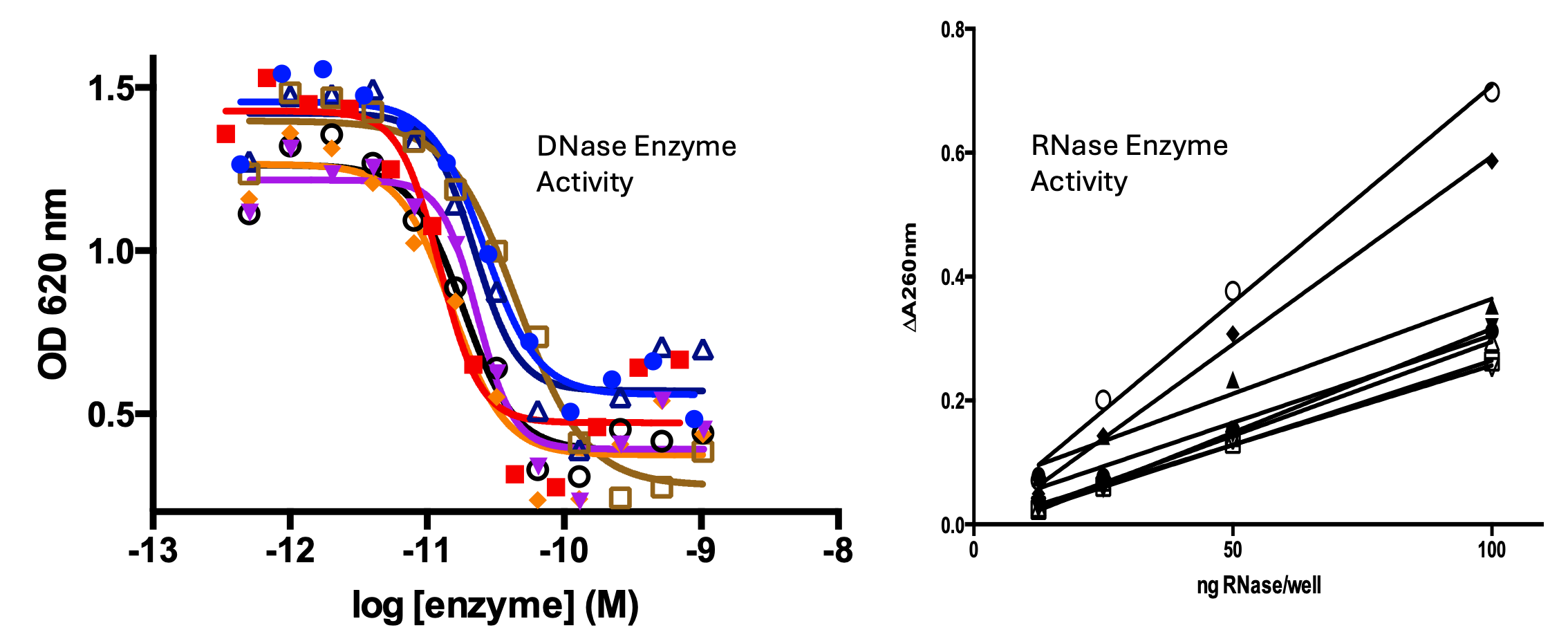
RSLV-132 has potent RNase catalytic enzyme activity combined with a three-week serum half-life and is engineered to remain in the plasma compartment where it digests pathogenic cell-free RNA.
RSLV-145
RSLV-145 is Resolve’s prototypical bi-specific nuclease Fc. This platform combines engineered hyper-active DNase enzyme activity with potent RNase enzyme activity on a clinically validated Fc domain. The molecules are designed to digest inflammatory NET nucleic acid scaffolds
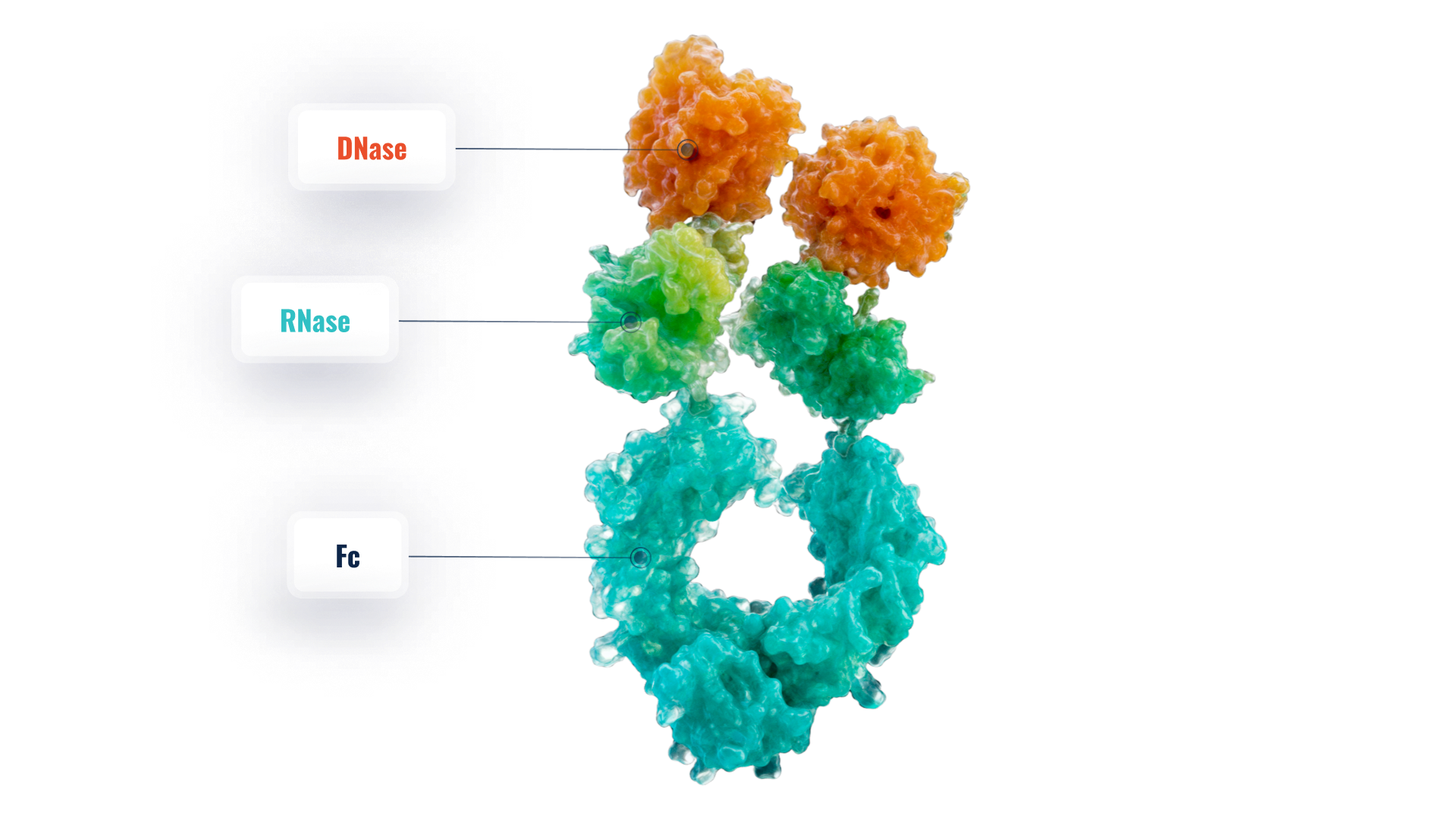
Resolve’s Bi-specific Nuclease Platform Combines Potent DNase catalytic activity with Robust RNase Catalytic Activity in a Single Fc Fusion protein