Resolve Therapeutics Announces Publication of Lupus Clinical Trial Results
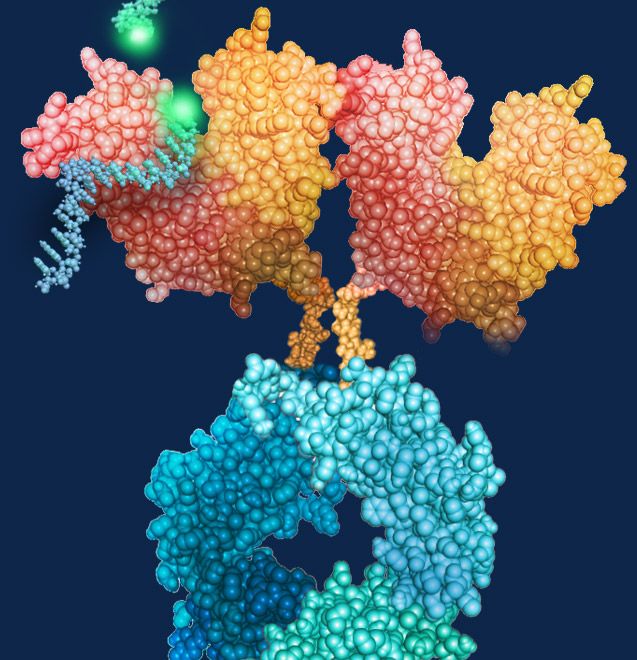
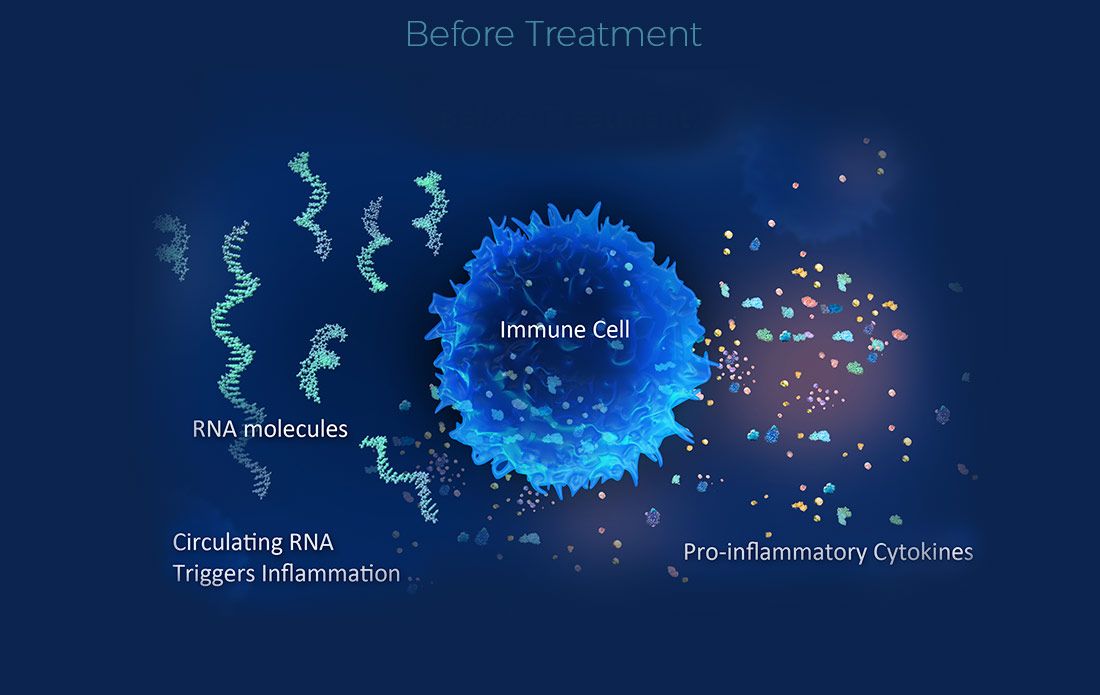
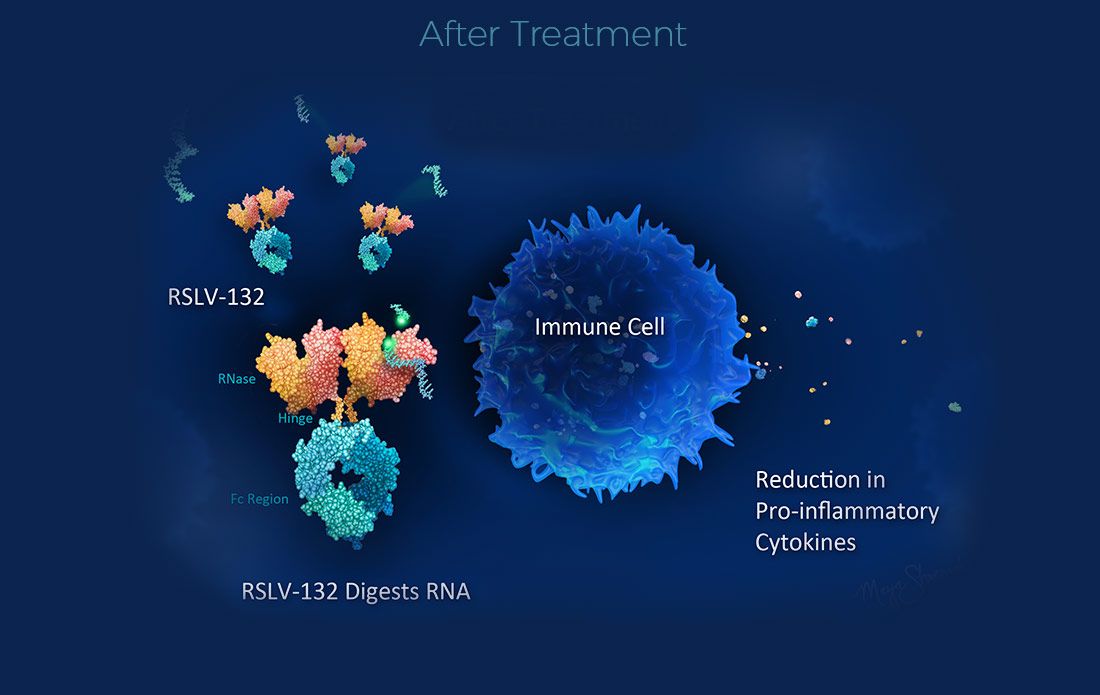
Miami, FL – February 12, 2024 – Resolve Therapeutics, a mid-stage clinical development biotechnology company pioneering first-in-class therapies for autoimmune diseases, today announced the publication of results from its phase 2a clinical trial of RSLV-132 in patients with Systemic Lupus Erythematosus (SLE) in Lupus Science & Medicine.